How Many Valence Electrons Does a Magnesium Atom Have
Magnesium has twelve valance electrons meaning its an atom with a metallic bonding and quite reactive. This element will loose 2 electrons to form ion.
How Many Electrons Does Magnesium Have Quora
The first electron shell of a Bohr model holds 2 electrons.

. Thus it has 2 valence electrons. 5 How many protons are in nitrogen-15. Magnesium has 12 protons and also 12 electrons.
Its electronic configuration is 282. Two valence electrons are present in Group 2s element. Well it is a Group 4 element meaning that it has electrons in the s p and d sublevels.
Magnesium has two valence electrons. 9 How many protons neutrons and electrons are there in nitrogen ion. Two valence electrons are present in Group 2s element.
For magnesium valency is same to the variety of electrons two in valence shell. 7 How many protons neutrons electrons are in nitrogen. How many valence electrons does magnesium have.
The full number ofelectrons current in the valence covering of one atom are referred to as valence electronsand there room a total of two electrons present in the valence shell of magnesium 3s2. We know that the valence electrons in magnesium Mg are two. Two valence electrons How.
Whats the distinction within the variety of electrons between the magnesium ion and elemental magnesium. The valence shell is the outermost shell of the atoms electrons. Magnesium has a atomic number of 12 and has a total.
What is the Bohr design for magnesium. That is the simple answer. Thus magnesium has two valence electrons.
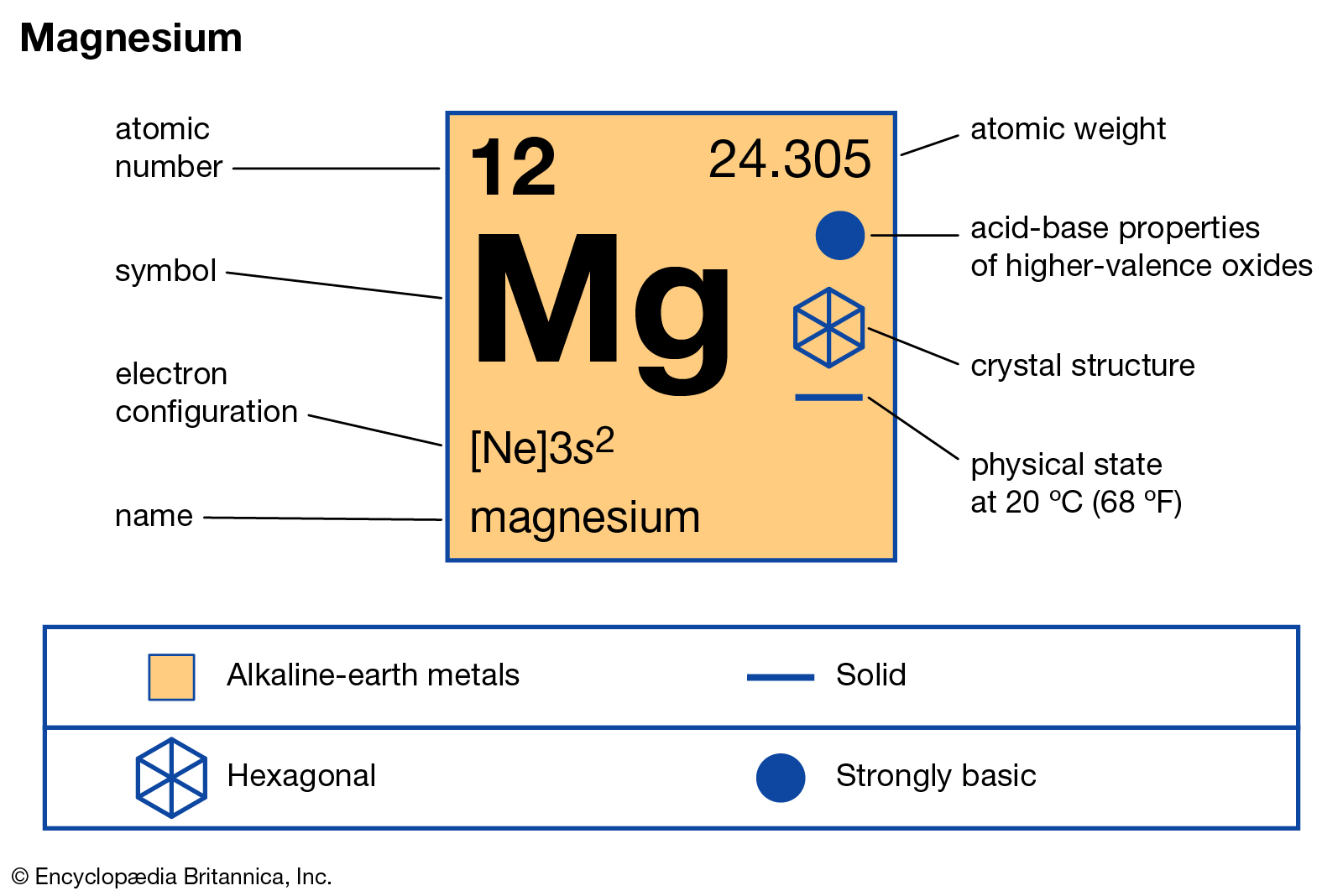
By signing up youll get thousands of step-by-step solutions to your homework. How many electron does magnesium have. Mgs electron configuration is also known as Ne3s-Does Magnesium Have 12 Electrons.
Its electrically impartial with 2 electrons in its outer valence shell. 6 How many protons and neutrons combined are in a nitrogen atom. Since the last shell of a zirconium ion has eight electrons the valence electrons of the zirconium ion Zr 4 are eight.
Magnesium has two valence electrons. Magnesium is a form 12 and is classified in Group 2 of the Periodic Table. This is because it can form bonds to some elements like oxygen or sulfur while also releasing hydrogen gas when exposed to water.
How many valence electrons does Mg2 have. A magnesium atom has 2 electrons in its valence shell. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of two electrons present in the valence shell of magnesium 3s 2.
Since it has 12 protons the atomic number is 12 and we know that the element with atomic number 12 is magnesium Mg. Magnesium has a total of 12 electrons 2 in the innermost shell 8 in the second shell and two electrons in its valence shell third shell. Number of valence electron in magnesium element 2.
Magnesium has a total of 12 electrons 2 in the innermost shell 8 in the second shell and two electrons in its valence shell third shell. Magnesium ion has 8 electrons in its valence shell. What has 12 protons and 12 neutrons.
The valence electrons may participate in bonding through sharing with other atoms to make three bonds. Magnesium is a form 12 and is classified in Group 2 of the Periodic Table. For oxygen valency is same to eight minus the number of electrons existing in the outermost shell 862.
Two of them are core electrons and the remaining 3 are valence electrons. How many valence electrons are there for magnesium. Three bonds six electrons.
Aluminum gallium indium and thallium have three electrons in their outermost shell a full s orbital and one electron in the p orbital with the valence electron configuration ns 2 np 1. 8 How many protons and neutrons does nitrogen 16 have. How many valence electrons does magnesium have.
It loses its two electrons to get stable or octet. Since the last shell of a magnesium-ion has eight electrons the valence electrons of magnesium ionMg 2 are eight. How many valence electrons does a neutral strontium atom have.
Magnesium is the 12th element of the periodic table having electronic configuration of. Does Magnesium Have 10 Valence Electrons. The electron configuration shows that the zirconium ion has acquired the electron configuration of krypton.
So magnesium is element number 12 in the periodic table. It has two free valence electrons which are used to bond with other atoms that have two valence electrons commonly oxygen or nitrogen. Magesiums common atomic mass is 24305 atomic mass models however no magnesium atom has precisely this mass.
4 How many electrons does an atom of nitrogen have. The most common and stable type of magnesium atom found in nature has 12 protons 12 neutrons and 12 electrons which have a negative charge. Therefore Magnesium atom has 2 valence electrons which explains why it likes to form Mg2 cations by losing the latter 2 electrons.
Hence the given element is a metal and the number of. 2 valence electrons Magnesium has atomic number 12. How many electrons are in indium outer shell.
Compound formation of magnesium Mg Magnesium participates in the formation of bonds through its valence electrons. Magnesium acquires a full octet by losing 2 electrons and emptying out its outermost shell. Mg is an atom of the component magnesium.
Mgs O2g MgOs or simply 2. Since the last shell of a magnesium-ion has eight electrons the valence electrons of magnesium ion Mg 2 are eight.
Electron Structure Of Magnesium

Valence Electrons For Magnesium Mg Youtube

How Many Valence Electrons Does Magnesium Mg Have Valency Of Magnesium
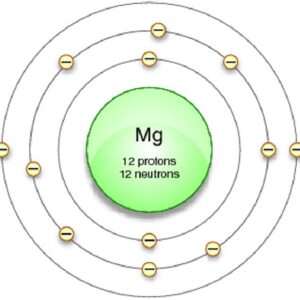
Magnesium Valence Electron Magnesium Valency Mg With Dot Diagram

1 Ionic And Metallic Bonding Ch Review What Is A Valence Electron Electrons In The Highest Outermost Occupied Energy Level Related To The Group Ppt Download
Magnesium Valence Electron Magnesium Valency Mg With Dot Diagram
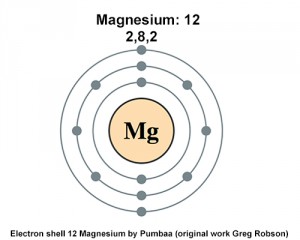
Magnesium Has 12 Protons How Many Electrons Are In Its First Energy Level Socratic

Magnesium Mg Electron Configuration And Orbital Diagram

How Many Valence Electrons Does Magnesium Mg Have
Chem4kids Com Magnesium Orbital And Bonding Info

The Number Of Electron Levels In A Magnesium Atom Is At Level
The Number Of Electron Levels In A Magnesium Atom Is At Level

Valence Electrons And Ions Periodic Table Of The Elements Ppt Download

Magnesium Bohr Model How To Draw Bohr Diagram For Magnesium Mg

The Number Of Electron Levels In A Magnesium Atom Is At Level

The Number Of Electron Levels In A Magnesium Atom Is At Level

How Many Valence Electrons Does Magnesium Mg Have
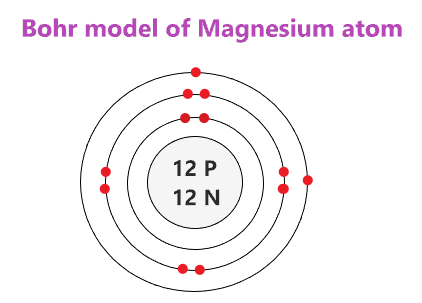
Magnesium Bohr Model How To Draw Bohr Diagram For Magnesium Mg
Comments
Post a Comment